R&D Towards better quality of life, GNT Pharma
Pipeline
Global New Drug Development
Pipeline
NELONEMDAZ Nelonemdaz / Drug for stroke
Overview
-
Drag from side to side.
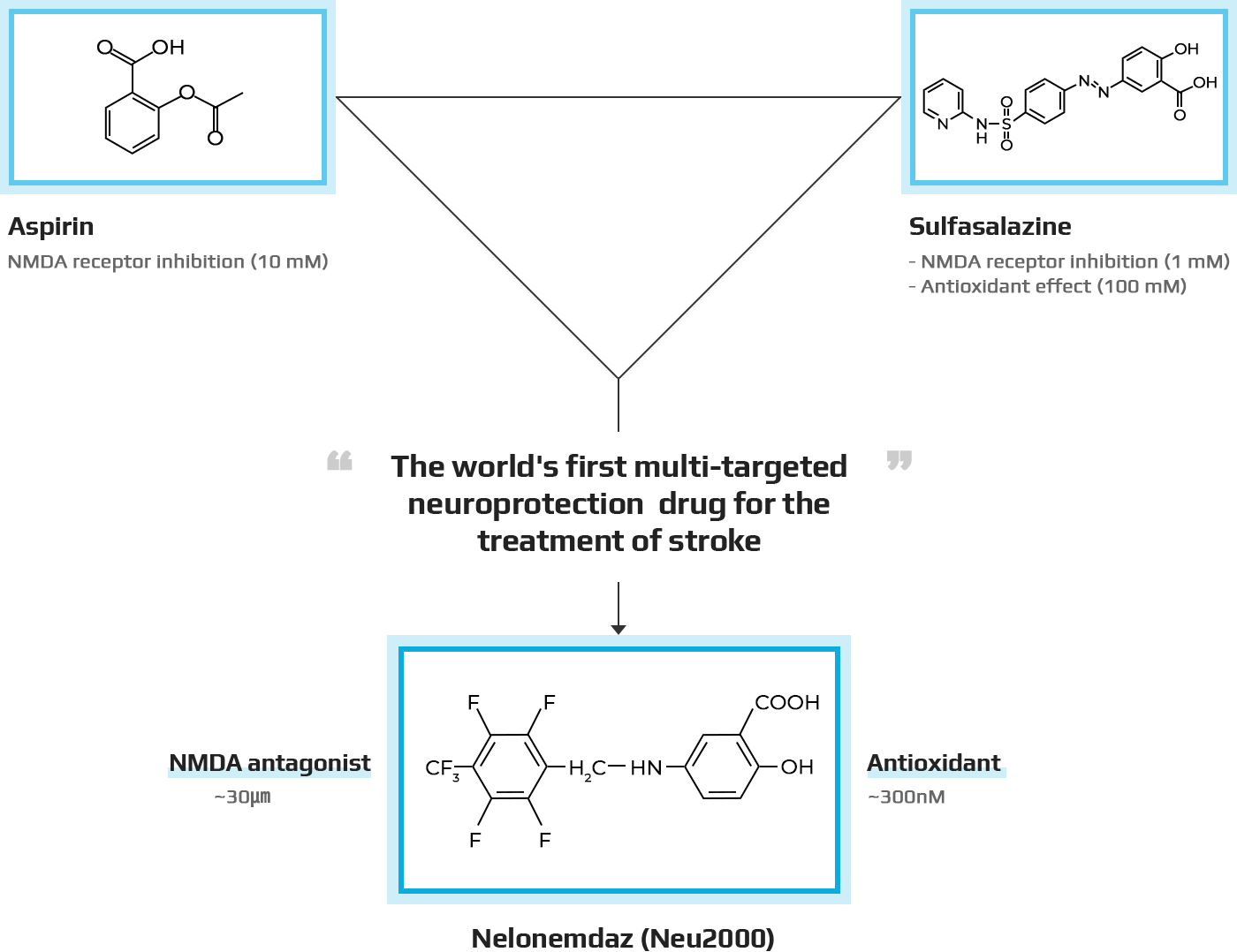
- Nelonemdaz is the first "multi-target" neuroprotection drug designed to reduce brain damage after stroke or cardiac arrest.
- Pharmacological action: (1) Inhibition of NMDA receptor subtype NR2B activity (2) Removal of free radicals (antioxidant)
- Unlike non-selective NMDA receptor inhibitors, nelonemdaz is a proven safe drug that does not cause adverse drug effects such as psychoses in normal people and stroke patients.
- Verification of safety and efficacy in the phase II clinical study (SONIC) in South Korea with 208 stroke patients undergoing a standard surgery to remove a blood clot.
- Verification of safety and efficacy in the phase II clinical study (ENIS) in China with 238 stroke patients receiving thrombolytics.
- Based upon the first interim analysis with 227 stroke patients of the phase III clinical study (ENIS III) in China, the IDMC (Independent data monitoring committee) recommended the continuation of the trial.
- Due to the long-lasting COVID-19 pandemic in China, ENIS-3 was terminated early in January 2024.
- In the Korean Phase III clinical trial (RODIN) involving 496 stroke patients who underwent thrombectomy within 12 hours of stroke onset, significant results were obtained in the group administered Nelonemdaz within 60 minutes of arrival at the emergency room.
Indications
- Stroke
- Cardiac Arrest
- Traumatic Brain Injury & Traumatic Spinal Cord Injury
- Burn Injuries
Comparison Safety and Efficacy of Nelonemdaz and clinical trial drugs for stroke
-
Drag from side to side.
| Type of stroke | Nelonemdaz | NMDA antagonists | Antioxidants | |
|---|---|---|---|---|
| Treatment Time Window in vivo | Severe tMCAO | 8 hour | Less than 1 hour | 2-4 hour |
| Mild tMCAO | 48 hour | ND (Not Determined) |
||
| Permanent MCAO | 4 hour | |||
| Forebrain ischemia | 24 hour | |||
| Safety in human | Phase I for Healthy Subjects Phase II for Stroke |
*Safe up to 6,000mg(IV) | SAE at target doses |
SAE at target doses (e.g. edaravone) |
| Two Phase I Studies 165 subjects in US & China Two Phase II Studies 447 patients in China & SK |
||||
| Efficacy in human | Ischemic Stroke alone | ND | All failed | All failed except edaravone approval in Japan |
| Ischemic stroke with thrombolytics | A phase III clinical study (ENIS III) was planned for 948 patients in China; IDMC (Jul 2022) recommended the continuation of the trial based upon interim analysis with 227 patients. | ND | ||
| Due to COVID-19 pandemic, ENIS III was terminated (Jan 2024) | ND | |||
| Ischemic stroke with endovascular thrombectomy | Promising beneficial effects in PII SOINC (N=209) | Nerinetide little beneficial effects in PIII (N=1105) | ND | |
| Significant results were obtained from stroke patients who received the drug within 60 minutes of arriving at the emergency room | ||||
| Cardiac Arrest with ROSC | AWAKE trial was completed; Nelonemdaz reduced disability and death | ND | ND | |
Related Articles
-
Journal of Cerebral Blood Flow and Metabolism. 2007
Download -
Journal of Neurochemistry. 2009
Download -
Drug News & Perspectives. 2010
Download -
Acta Neuropathol. 2010
Download -
Journal of Neurotrauma. 2010
Download -
Experimental and Molecular Medicine. 2011
Download -
Toxicology in Vitro. 2013
Download -
Archives of Phamacal Research. 2013
Download -
Trials. 2018
Download -
Trials. 2022
Download -
Stroke. 2022
Download
